AYVAKIT® (avapritinib) is indicated for the treatment of adult patients with indolent systemic mastocytosis (ISM).
Limitations of Use: AYVAKIT is not recommended for the treatment of patients with ISM with platelet counts of <50 x 109/L.
Cognitive Effects—Cognitive adverse reactions can occur in patients receiving AYVAKIT and occurred in 7.8% of patients with ISM who received AYVAKIT + best supportive care (BSC) versus 7.0% of patients who received placebo + BSC; <1% were Grade 3. Depending on the severity, withhold AYVAKIT and then resume at the same dose, or permanently discontinue AYVAKIT.
Photosensitivity—AYVAKIT may cause photosensitivity reactions. In all patients treated with AYVAKIT in clinical trials (n=1049), photosensitivity reactions occurred in 2.5% of patients. Advise patients to limit direct ultraviolet exposure during treatment with AYVAKIT and for one week after discontinuation of treatment.
Embryo-Fetal Toxicity—AYVAKIT can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females and males of reproductive potential to use an effective contraception during treatment with AYVAKIT and for 6 weeks after the final dose. Advise women not to breastfeed during treatment with AYVAKIT and for 2 weeks following the final dose.
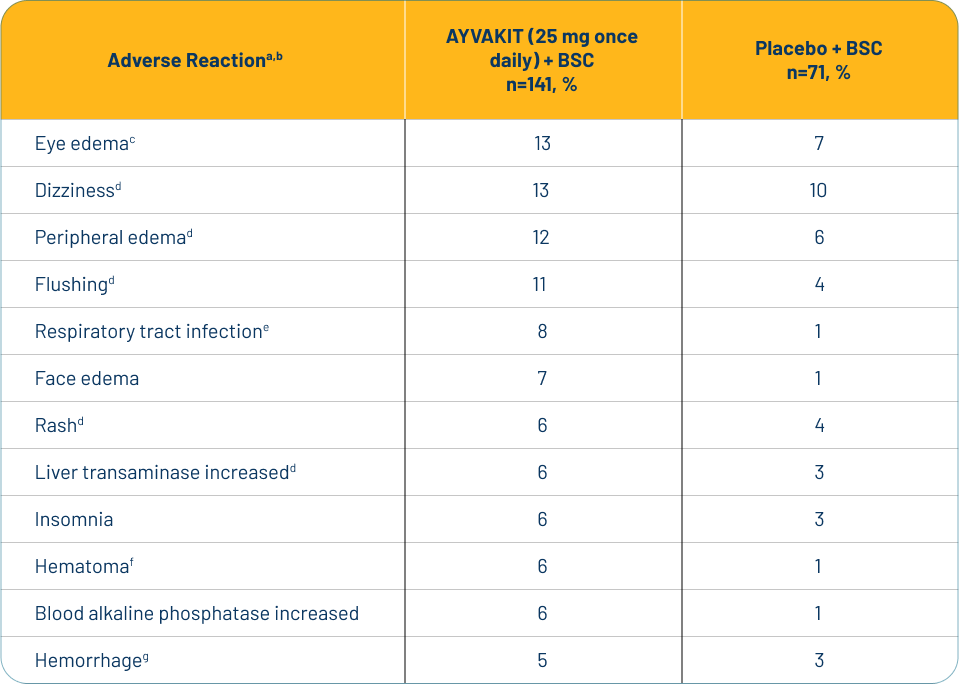
Adverse Reactions—The most common adverse reactions (≥10%) in patients with ISM were eye edema, dizziness, peripheral edema, and flushing.
Drug Interactions—Avoid coadministration of AYVAKIT with strong or moderate CYP3A inhibitors or inducers. If contraception requires estrogen, limit ethinyl estradiol to ≤20 mcg unless a higher dose is necessary.
To report suspected adverse reactions, contact Blueprint Medicines Corporation at 1-888-258-7768 or the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
Please click here to see the full Prescribing Information for AYVAKIT.
References:
- AYVAKIT [prescribing information]. Cambridge, MA: Blueprint Medicines Corporation; November 2024.
- Kristensen T et al. Am J Hematol. 2014;89(5):493-498.
- Garcia-Montero AC et al. Blood. 2006;108(7):2366-2372.
- Ungerstedt J et al. Cancers. 2022;14(16):3942.
- Pardanani A. Am J Hematol. 2023;98(7):1097-1116.
- Data on file. Blueprint Medicines Corporation, Cambridge, MA. 2023.
- Gülen T et al. J Intern Med. 2016;279(3):211-228.
- Theoharides TC et al. N Engl J Med. 2015;373(2):163-172.
- Gotlib J et al. NEJM Evidence. 2023;2(6). Published online May 23, 2023. doi:10.1056/EVIDoa2200339
- Gilreath JA et al. Clin Pharmacol. 2019;11:77-92.
- Evans EK et al. Sci Transl Med. 2017;9(414):eaao1690.
- Padilla B et al. Orphanet J Rare Dis. 2021;16(1):434.
- van Anrooij B et al. Allergy. 2016;71(11):1585-1593.
- WHO Classification of Tumours Editorial Board. Haematolymphoid tumours [Internet]. Lyon (France): International Agency for Research on Cancer; 2024 [cited April 24, 2024]. (WHO Classification of Tumours Series, 5th ed.; vol. 11). Available from: https://tumourclassification.iarc.who.int/chapters/63
- Dranitsaris G et al. J Oncol Pharm Pract. Published online December 27, 2023. doi:10.1177/10781552231221149
- Siebenhaar F et al. Immunol Allergy Clin North Am. 2014;34(2):433-447.
- Jennings SV et al. Immunol Allergy Clin North Am. 2018;38(3):505-525.
- Akin C, ed. Mastocytosis: A Comprehensive Guide. Springer; 2020.