YourBlueprint® provides dedicated, personalized support to help your patients from Day 1
YourBlueprint is a patient support program designed with your patients in mind. YourBlueprint provides a variety of support to eligible patients throughout many aspects along the treatment journey.
- Co-pay Support
- Coverage Interruption
- QuickStart
- Patient Assistance Program
- Case Managers can also help your patients through nonclinical aspects of therapy by providing 1:1 support calls and patient education resources
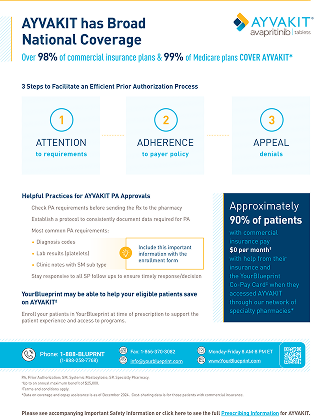
AYVAKIT has broad national coverage
Over 98% of commercial insurance plans and 99% of Medicare plans cover AYVAKIT*
*Data on coverage are as of September 2025.
Co-pay Assistance Program
Approximately 90% of patients with commercial insurance paid $0 per month† with help from their insurance and with the YourBlueprint® Co-Pay Card‡ when they accessed AYVAKIT through our network of specialty pharmacies.§
†Data on co-pay assistance are as of December 2025. Cost-sharing data are for those patients with commercial insurance.
‡Up to an annual maximum benefit of $25,000. Terms and conditions apply.
§This program covers co-pay, co-insurance and deductible expenses for those who qualify.
Enroll your patients at the time of prescription to support the patient experience and access to programs
Enroll your patientAYVAKIT will require a prior authorization with
the enrollment form
Most denials for AYVAKIT are due to missing or incomplete information. When working on insurance coverage approval for AYVAKIT, YourBlueprint and our network of specialty pharmacies can help support your patient through the process of managing a prior authorization requirement.
AYVAKIT prior authorization requirements with the enrollment form:
Diagnosis codes
Lab results
(platelet counts, per label)
Clinic notes with
SM subtype
Access and Support Resources
YourBlueprint Program Overview for HCPs
A high-level overview of the YourBlueprint patient support program for HCPs.
Download Program OverviewAccess and Coverage Flashcard
A resource showing the cost and coverage information on AYVAKIT.
Download the FlashcardHCP=healthcare provider; SM=systemic mastocytosis.
Looking for additional resources for your practice and your patients?
PRACTICE RESOURCESPatient portrayal