ISM is an overproduction of mutated and hyperactive mast cells creating disruption throughout the body1,2
~95% of ISM cases involve the KIT D816V mutation3-5
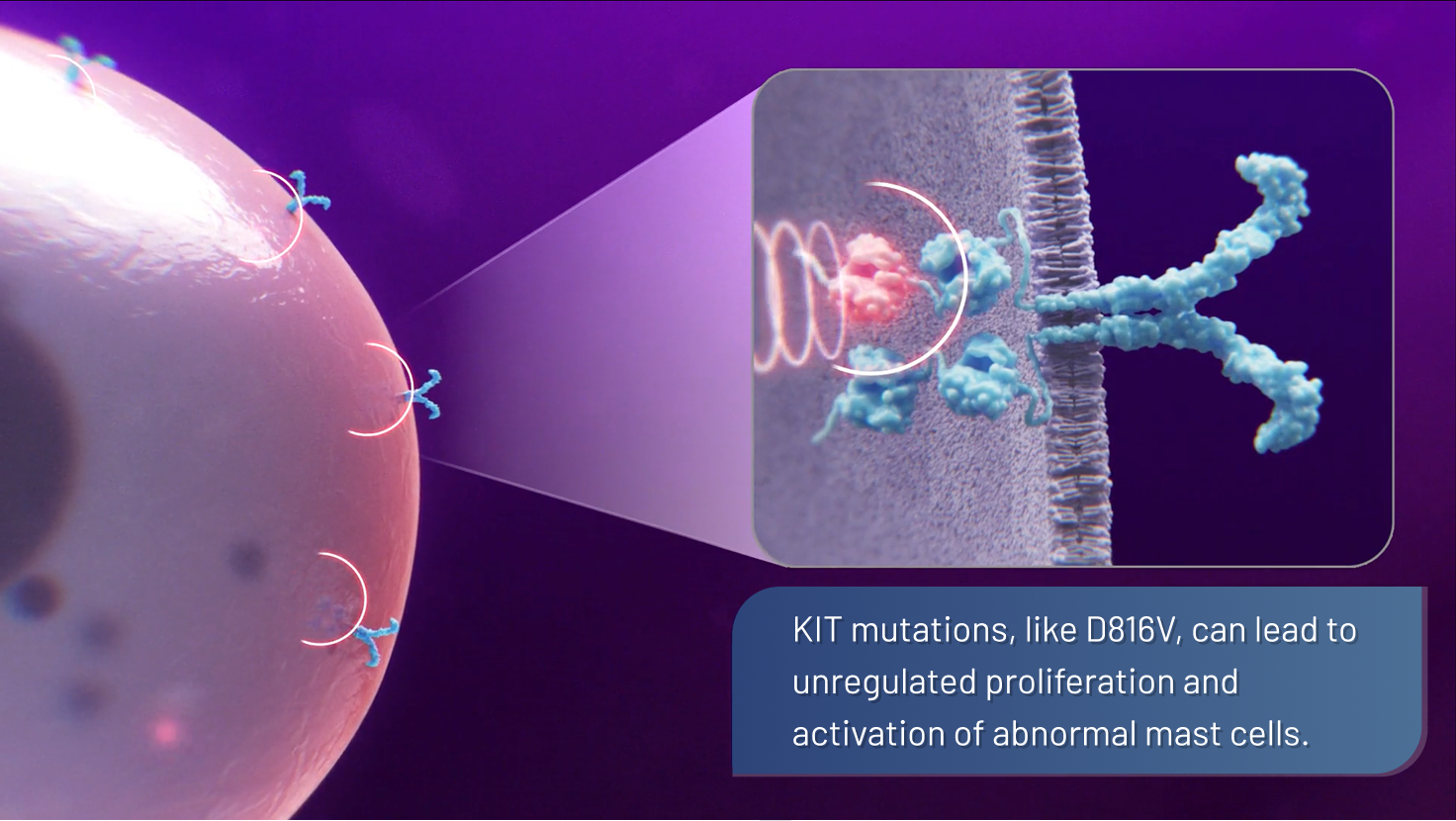
- This mutation causes the KIT receptor to be constantly “switched on,“ disrupting normal cell growth regulation, leading to the overproduction of mutated, hyperactive mast cells1,2,6,7
- These mutated, hyperactive mast cells then proliferate, accumulate, and release mediators that can trigger potentially debilitating symptoms across organ systems1,2,8
Symptoms can flare unexpectedly and are triggered by
aspects of everyday life1,2,8
These symptoms represent the clinical spectrum of ISM. Symptoms may vary in individual patients.
65%
of patients reported experiencing nervous system disorders 9*
80%
of patients reported experiencing GI disorders 9*
76%
of patients reported experiencing skin and subcutaneous tissue disorders 9*
* Data based on ongoing medical history events in the PIONEER study (N=212). Events ≥10% in 1 or both trial arms from 3 of the organ classes include pruritus, urticaria pigmentosa, urticaria, rash maculo-papular for skin and subcutaneous tissue disorders; headache, dizziness, migraine, memory impairment, disturbance in attention for nervous system disorders; and diarrhea, abdominal pain, nausea, gastroesophageal reflux disease, abdominal distension, constipation for gastrointestinal disorders.9
GI=gastrointestinal; ISM=indolent systemic mastocytosis; KIT=KIT proto-oncogene, receptor tyrosine kinase.

References: 1. Pardanani A. Am J Hematol. 2023;98(7):1097-1116. 2. Gülen T et al. J Intern Med. 2016;279(3):211-228. 3. Kristensen T et al. Am J Hematol. 2014;89(5):493-498. 4. Garcia-Montero AC et al. Blood. 2006;108(7):2366-2372. 5. Ungerstedt J et al. Cancers. 2022;14(16):3942. 6. Valent P et al. Blood. 2017;129(11):1420-1427.7. da Silva EZM et al. J Histochem Cytochem. 2014;62(10):698-738. 8. Akin C, ed. Mastocytosis: A Comprehensive Guide. Springer; 2020. 9. Data on file. Blueprint Medicines Corporation, Cambridge, MA.
