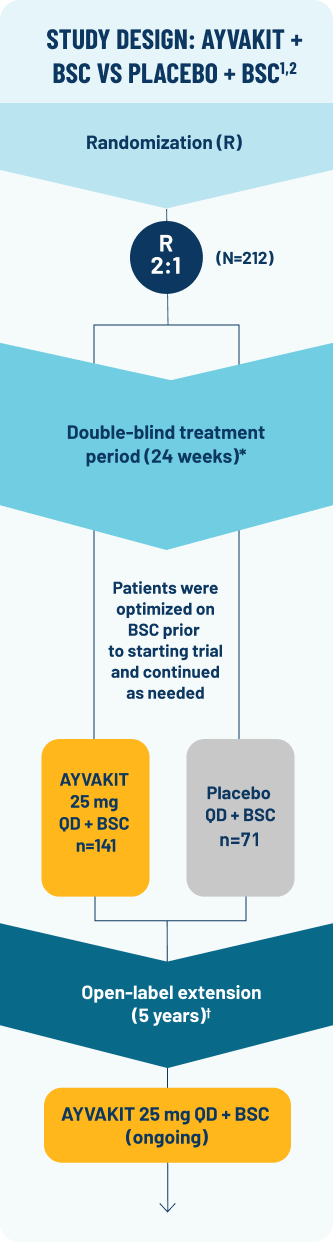
PIONEER was designed to measure symptom relief and change in mast cell burden1,2
PIONEER: A phase 2, multipart, randomized, placebo-controlled, double-blind trial (N=212) evaluating the efficacy and safety of AYVAKIT 25 mg vs placebo at 24 weeks, both arms receiving concomitant BSC1,2
Key eligibility criteria: ≥18 years of age; centrally confirmed ISM diagnosis per WHO criteria; uncontrolled moderate to severe ISM symptoms (defined as ISM-SAF TSS ≥28) despite ≥2 BSC2
Best supportive care (BSC) is usually referred to as
symptom-directed therapies in clinical settings
SYMPTOM MEASUREMENT
- Primary endpoint: Absolute mean change in ISM-SAF TSS compared with placebo + BSC from baseline to Week 241
- Exploratory endpoints: Mean change in ISM-SAF individual symptom scores; mean change in most severe symptom score at Week 242,3
MAST CELL BURDEN MEASUREMENT
- Select key secondary endpoints compared with placebo + BSC at Week 241,2:
- ≥50% reduction in serum tryptase levels
- ≥50% reduction in KIT D816V VAF or undetectable‡
- ≥50% reduction in bone marrow mast cells or no aggregates
- Proportion of patients achieving:
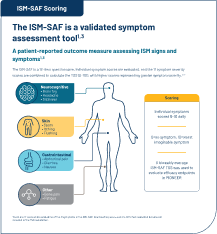
ISM-SAF TSS
The ISM-SAF is a validated patient-reported outcome measure assessing 11 ISM signs and symptoms (abdominal pain, nausea, diarrhea, spots, itching, flushing, bone pain, fatigue, dizziness, headache, and brain fog)2§
*Data cutoff was June 23, 2022.2
†Patients had the option to enter part 3 of PIONEER, an open-label extension evaluating the long-term efficacy and safety of AYVAKIT 25 mg + BSC for up to 5 years. All eligible patients either
continued AYVAKIT 25 mg + BSC daily or switched from placebo + BSC to AYVAKIT 25 mg + BSC.2
‡In peripheral blood.1
§Symptom severity scores (scored 0 [no symptoms] to 10 [worst imaginable symptoms] daily) are combined to calculate the TSS from 0-110, with higher scores representing greater symptom
severity. A biweekly average in ISM-SAF TSS was used to evaluate efficacy endpoints.1,4
BSC=best supportive care; ISM=indolent systemic mastocytosis; ISM-SAF=Indolent Systemic Mastocytosis-Symptom Assessment Form; KIT=KIT proto-oncogene, receptor tyrosine kinase;
QD=every day; TSS=total symptom score; VAF=variant allele fraction; WHO=World Health Organization.
PIONEER reflects a heterogenous population of patients living with ISM2
Select baseline demographics and patient characteristics (AYVAKIT + BSC, n=141; placebo + BSC, n=71)1,2
Patients were optimized on a range of BSC2
- Anti-immunoglobulin E antibody (omalizumab)
- Glucocorticoids
- Cromolyn sodium
- H1 antihistamines
- H2 antihistamines
- Leukotriene inhibitors
- Proton pump inhibitors
93% of patients in the AYVAKIT + BSC arm were KIT positive, with 7% KIT undetectable1

References: 1. AYVAKIT [prescribing information]. Cambridge, MA: Blueprint Medicines Corporation; November 2024. 2. Gotlib J et al. NEJM Evidence. 2023;2(6). Published online May 23, 2023. doi:10.1056/EVIDoa2200339 3. Data on file. Blueprint Medicines Corporation, Cambridge, MA. 4. Padilla B et al.Orphanet J Rare Dis. 2021;16(1):434.
