GUIDELINES®
RECOMMENDED
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend avapritinib (AYVAKIT) (if platelets ≥50 x 109/L) as an NCCN Category 2A, preferred first-line treatment option for advanced systemic mastocytosis (SM), including aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN) (when the SM component requires prioritization over the AHN component), and mast cell leukemia (MCL).2*
*NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN=National Comprehensive Cancer Network.
(95% CI: 42%, 70%)†
Proven efficacy and demonstrated duration of response1
38.3-month median duration of response (DOR) demonstrated in clinical trials (95% CI: 19, NE) DRIVEN PRIMARILY by SM-AHN patients1
EXPLORER and PATHFINDER were 2 multicenter, single-arm, open-label clinical trials evaluating the efficacy and safety of AYVAKIT for patients with Advanced SM.1
Efficacy was based on overall response rate (ORR) per modified IWG-MRT-ECNM criteria across all evaluable patients dosed up to 200 mg daily (N=53). In the subgroup of patients with MCL, the efficacy was based on complete remission (CR).1
53 patients were evaluable for a response across the 2 trials, with a median follow-up of 11.6 months (95% CI: 9.9 to 16.3 months)1
Patients enrolled in EXPLORER received a starting dose of AYVAKIT ranging from 30 mg to 400 mg orally once daily. In PATHFINDER, patients were enrolled at the recommended starting dose of 200 mg orally once daily1‡
†ORR per modified IWG-MRT-ECNM is defined as patients who achieved a CR, CRh, or PR.
‡The recommended dosage of AYVAKIT for patients with AdvSM is 200 mg.
View criteria for response-evaluable patients1
View baseline demographic characteristics (N=53)1
Proven efficacy across all subtypes and regardless of prior antineoplastic therapy1,3
Proven efficacy and demonstrated duration of response in Advanced SM1
ORR across all evaluable patients with Advanced SM (N=53)§ who were dosed up to 200 mg daily1,3
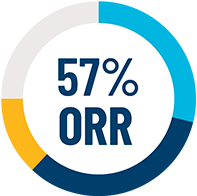
(95% CI: 42%, 70%)¶
72% ORR was achieved with the addition of patients who had clinical improvement||
¶ORR per modified IWG-MRT-ECNM is defined as patients who achieved a CR, CRh, or PR. Modified IWG criteria evaluated overall response rate by ≥12 weeks’ response duration, resolution of ≥1 findings of nonhematologic and hematologic organ damage (C-findings), and ≥50% reduction in mast cell burden and serum tryptase (must be <20 ng/mL if baseline was ≥40 ng/mL for CR/CRh).
§Median duration of follow-up was 11.6 months (95% CI: 9.9, 16.3).
∥Clinical improvement is defined as having a response duration of ≥12 weeks and fulfillment of 1 or more of the nonhematologic and/or hematologic response criteria4
Median DOR in
the overall population
38.3
MONTHS
(95% CI: 19, NE)1
Median time to
response
2.1
MONTHS
(n=30)1
Median time to
CR and CRh
9.2
MONTHS
(n=15)3
Proven efficacy across subtypes and regardless of prior antineoplastic therapy1
MCL (n=11)1
45% ORR
(95% CI: 17%, 77%)
SM-AHN (n=40)1
58% ORR
(95% CI: 41%, 73%)
78% ORR was achieved with the
addition of patients who had a
clinical improvement1
ASM (n=2)1
100% ORR
(95% CI: 16%, 100%)
1 of 2 patients
achieved CR/CRh1
In a preplanned subgroup analysis, AYVAKIT demonstrated efficacy regardless of prior antineoplastic therapy3
In treatment-naive patients (n=18), ORR
was 72.2% (95% CI: 46.5%, 90.3%)
In patients with prior antineoplastic therapy
(including midostaurin) (n=35), ORR was
48.6% (95% CI: 31.4%, 66%)
Individual results may vary.
Learn about the safety of AYVAKIT evaluated in the
EXPLORER and PATHFINDER clinical trials.
References: 1. AYVAKIT [prescribing information]. Cambridge, MA: Blueprint Medicines Corporation; November 2024. 2. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Systemic Mastocytosis V.1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed February 21, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 3. Data on file. Blueprint Medicines Corporation, Cambridge, MA. 4. Gotlib J et al. Blood. 2013;121(13):2393-2401.
